End of the bedrest campaign
ESA PR 54-2002. Long-duration spaceflight has a significant impact on the body, with astronauts experiencing changes in their bone and muscle in particular. To meet the requirements of long-stay missions aboard the International Space Station and prepare for future interplanetary missions, space agencies are working together to develop preventive-medicine methods or countermeasures intended to overcome the adverse effects of spaceflight.
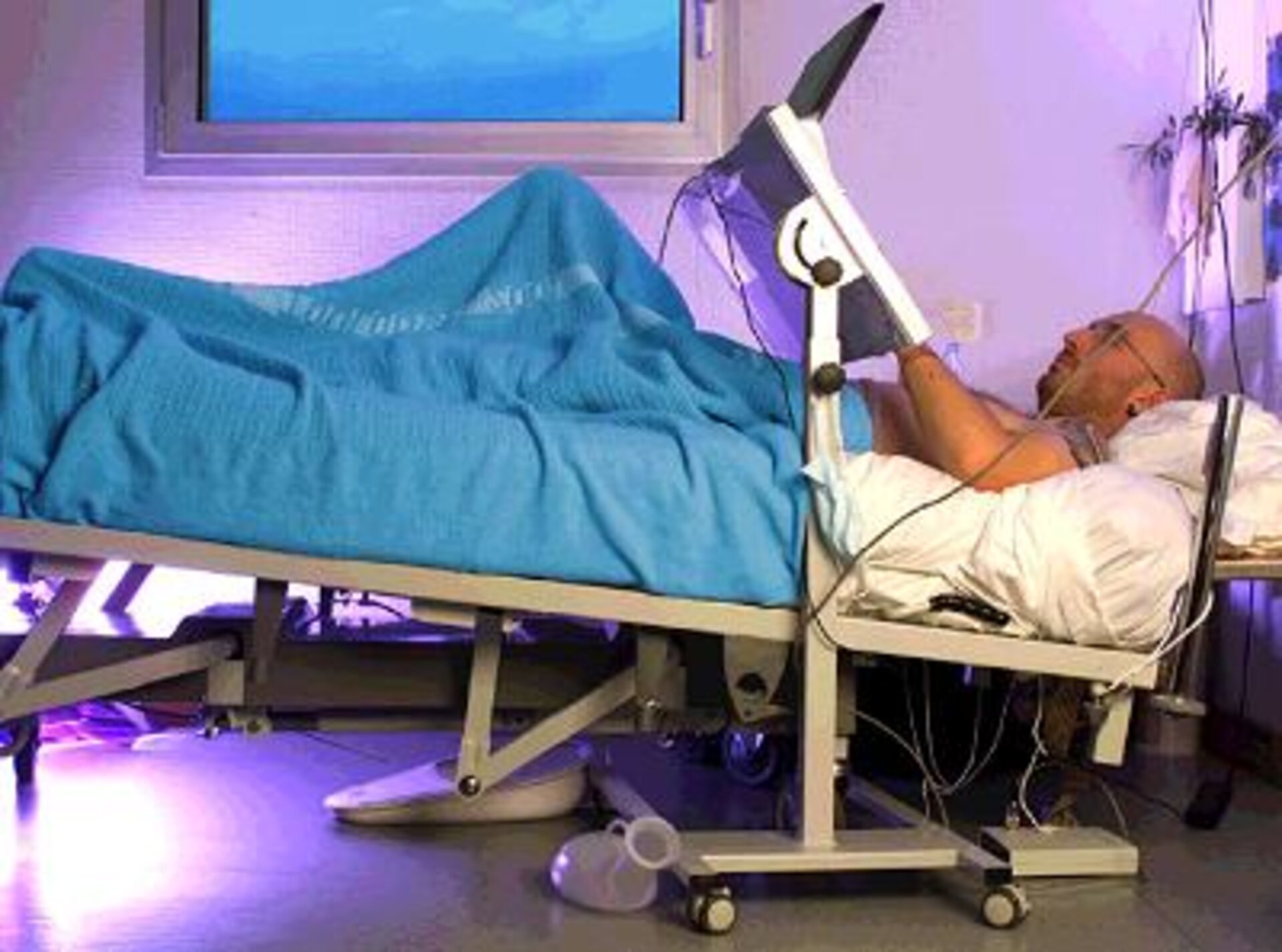
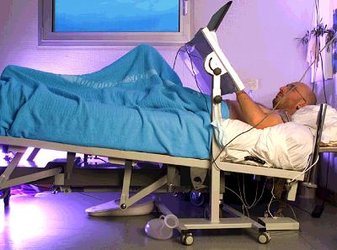
The European, French and Japanese space agencies (ESA, CNES and NASDA, respectively) set up the long-term bedrest (LTBR) experiment to simulate on the ground the effects of long-duration weightlessness. It was based on the anti-orthostatic (-6° head-down tilt) bedrest model (see ESA press releases nos 46-2001, 60-2001, 74-2001 and 26-2002).
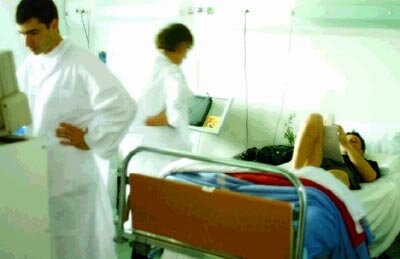
The study took place at the MEDES Space Clinic in Toulouse (France) over two four-month phases, the first from August to December 2001 and the second from March to July 2002. To ensure sound scientific interpretation of the study, a homogeneous group of male candidates aged between 25 and 45 was selected.
Out of a total of 725 applications received and following 123 medical examinations, 25 volunteers were chosen. They were all French, except for one Belgian, and aged between 26 and 41. Their occupations included history and geography teacher, builder, postman, gardener, book-keeper and mobile phone salesman.
Throughout the study, the volunteers underwent numerous examinations, such as stress response tests, bone densitometry and MRI. Muscle biopsies and biochemical analysis of blood and urine samples were also carried out. Special medical checkups are scheduled 45 days, 3 months, 6 months and 12 months after bedrest, to be supplemented by a questionnaire after 2 years. The 25 volunteers for the two phases successfully completed the experiment.
The initial results and conclusions of the study will be discussed at a scientific seminar followed by a press conference in Toulouse in January 2003.
To find out more about this study:
- consult website http://www.medes.fr
- contact those responsible for the study:
Benny Elmann-Larsen
ESA – Physiology experiment coordinator, HDT study project manager
Tel: +31 71 565 3517
Fax: +31 71 565 3661
Email: benny.elmann-larsen@esa.int
Antonio Guell
CNES – Head of life sciences programmes, HDT study programme committee member
Tel: +33 561 28 2577
Fax: +33 561 27 3091
Email: antonio.guell@cnes.fr
Eliane Moreaux
CNES – Communications officer
Tel: +33 05.61.27.33.44
Fax: +33 05.61.28.29.39
Email: eliane.moreaux@cnes.fr