
Growth Factor-Induced Signal Transduction in Mammalian Cells is
Sensitive to Gravity
J. Boonstra, P. J. Rijken & A. J. Verkleij
Department of Molecular Cell Biology, University of Utrecht,
Padualaan 8, NL-3584 CH Utrecht, The Netherlands
P. T. van der Saag, A. F. L. van Puijenbroek & S. W. de
Laat
Hubrecht Laboratory, National Institute of
Developmental Biology, Uppsalalaan 8, NL-3584 CT Utrecht, The
Netherlands
Epidermal growth factor (EGF) activates a well-
characterised signal transduction cascade in human A431
epidermoid carcinoma cells. This activation leads to increased
cell proliferation in most cell types. Among the early responses
evoked by EGF are receptor clustering, cell rounding and early
gene expression. The influence of gravity on EGF-induced EGF
receptor clustering and early gene expression, as well as actin
polymerisation, actin organisation and cell rounding have been
investigated.
EGF-induced c-fos and c-jun expression
decreased in microgravity. This was caused by the EGF receptor
and protein kinase C mediated signal transduction pathways, but
not by the Ca²+ or protein kinase A pathways. In
contrast, neither the binding of EGF to the receptor nor receptor
clustering was modulated under µg conditions. The relative
filamentous actin content was enhanced under µg and the
actin filament organisation was altered. Interestingly, the
microtubule and keratin organisation showed no difference under
µg.
Introduction
A number of studies have indicated that gravity affects
mammalian cell growth and differentiation.¹,² To study
the effect of variations in gravity on human cells at the
molecular level, the well-characterised epidermal growth factor
(EGF)-induced signal transduction in A431 epidermoid carcinoma
cells was used as a model system. EGF is a member of a family of
polypeptide growth factors, involved in the control of mammalian
cell growth and differentiation. It is a low molecular weight
polypeptide which enhances the proliferation of various cell
types both in vivo and in vitro. EGF acts by
binding to a specific transmembrane receptor. This receptor is
a glycoprotein with an extracellular EGF-binding domain and an
intracellular protein tyrosine kinase domain. EGF binding to the
receptor induces a dimerisation or clustering of part of the EGF
receptor population. Several studies have indicated that receptor
dimerisation leads to kinase activation. The subsequent signal
transduction pathways activated by EGF include the
phosphatidylinositol pathway and the ras pathway. These pathways
lead to the induction of early gene expression such as the proto-
oncogenesc-fos and c-jun. In addition, the EGF
receptor activation leads to an increase in actin polymerisation
and a drastic reorganisation of the actin microfilament system,
leading to a rounding-up of the cells.³
To determine the influence of µg on EGF-induced signal
transduction, experiments were performed during the MASER 3-6
flights, during which the effect of µg was studied on EGF-
binding, EGF receptor clustering, actin polymerisation, cell
morphology and early gene expression.
Materials and methods
Cell culture
A431 human epidermoid
carcinoma cells were grown in Dulbecco modified essential medium
(DMEM) supplemented with 7.5% foetal calf serum (FCS) in a 7%
humidified atmosphere. For the experiments performed in the MASER
sounding rockets, the cells were cultured on coverslips and
mounted in Cells in Space (CIS) plunger box units as described
in ref. 10.
EGF binding studies
EGF binding was
determined by using 125 I-EGF (2 ng/ml) as described
in detail in ref. 4.
EGF receptor distribution
The EGF
receptor distribution was determined by the label fracture
method,5 using immunogold labelling as described in
detail in ref. 6.
Actin polymerisation and organisation The
actin polymerisation and organisation was studied using
fluorescent-labelled phalloidin with conventional and confocal
laser scanning microscopy, as described in detail in refs. 6 &
7.
Early gene expression
The expression of
c-fos and c-jun was determined by the RNAse
protection assay as described in ref. 9.
Results and discussion
Early gene expression
An excellent end-
point to monitor possible gravity effects on EGF-induced signal
transduction is provided by the EGF-induced expression of so-
called immediate early genes. These genes are the first to be
transcribed after stimulation of cells with growth factors such
as EGF. Examples are the c-fos, c-jun and c-myc
genes.
Experiments performed during the MASER 3-4 flights
demonstrated that µg suppressed EGF-induced c-fos and
c-jun expression (Fig. 1). On the other hand, the
expression of the constitutively expressed ß-2 microglobulin
gene, which is not modulated by EGF, remains
unaffected.9 Quantitative analysis shows that c-fos
and c-jun expression in µg is reduced by
approximately 50% compared to the normal gravity control samples.
These data demonstrate that µg inhibits expression that is
specific for EGF-induced signal transduction.
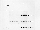
Fig. 1. Microgravity decreases EGF-induced c-fos and c-jun
expression. As soon as µg was attained, A431 cells were
treated with EGF (+EGF) for 6 min or with buffer alone (-EGF) for
0-6 min. To test for possible launch effects, gene expression was
determined in samples lysed at the beginning of the µg phase
(Flight, -EGF, 0 min). RNA was isolated and analysed for c-fos,
c-jun and ß-2 microglobulin expression.
EGF binding
One likely explanation for
the observed inhibition of gene expression may well be that
µg leads to decreased binding of EGF to the cell surface-
located EGF receptor. EGF binding was therefore studied during
the MASER 5-6 flights, using125 I-EGF. These
experiments clearly showed that µg did not influence the
binding properties of the receptors (Fig. 2). Therefore, we
conclude that µg inhibits EGF-induced signal transduction
downstream of the initial activation, i.e. the binding of EGF to
its receptor.
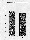
Fig. 2. 125 I-radiolabelled EGF was allowed to bind
to the cell surface EGF receptor population for 6 min (A). Cells
were subsequently fixed, lysed and bound EGF was determined in
cpm/g of cellular protein. A similar experiment was performed
under µg during MASER 5 (B).
EGF receptor distribution
One of the
first cellular responses following binding of EGF to the receptor
is a redistribution of the cell surface EGF receptor population,
leading to receptor clustering.5 Electron microscope
techniques allow direct viewing and quantitative analysis of the
receptor distribution at an ultrastructural level. It was
demonstrated during the MASER 3-4 flights that the receptor
distribution of cells in control and EGF-treated cells in µg
and under normal gravity were fully comparable (Fig. 3). Thus
µg influences EGF-induced signal transduction downstream of
EGF binding and EGF receptor redistribution, but upstream of
early gene expression.
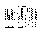
Fig. 3. The effect of µg on EGF-induced EGF receptor
clustering: a quantitative analysis. A431 cells were brought into
µg and fixed to detect launch effects. Other samples were
treated for 5 min with EGF or buffer alone under µg
conditions. After flight the cells were immunogold-labelled,
frozen and freeze-fractured. The mean percentage monomers of
cells from two independent experiments of launch control (0-),
EGF-treated (5+) and control (5-) cells were normalised according
to the regressions of the percentage monomers on the particle
density as described in detail in ref. 6.
Microgravity-sensitive signal transduction
pathways
The observations described so far indicate
that specific signalling pathways are affected by µg. This
could mean that µg affects a limited number of gravity-
sensitive cellular targets. The expression of c-fos and
c-jun genes is rapidly induced by growth factors, but it
can also be triggered by a variety of agents that mimic the
partial activation of signal transduction pathways but bypass the
EGF receptor. Examples of such agents are the phorbol ester PMA
(activates protein kinase C), the calcium ionophore A23187
(mimics the EGF-induced increase in the intracellular free
calcium concentration) and forskolin (raises the intracellular
cyclic AMP concentration).
During the MASER 4-5 flights, A431 cells were activated
respectively with EGF, PMA, A23187 and forskolin. Quantitative
analysis of the c-fos and c-jun expression
demonstrated that both EGF- and PMA-induced expressions were
strongly inhibited under µg, while the forskolin- and
A23187-induced gene expressions were not affected (Table 1). As
EGF and PMA are known to be activaters of PKC, these data
indicated that the cellular response to PKC-mediated signal
transduction is a molecular target for µg.10
Table 1. EGF-induced c-fos and c-jun expression in
µg. Early gene expression was determined by the RNAse
protection assay as described in ref. 10. The ratios of gene
expression under normal and µg conditions were determined.
NC: not calculated; ND: not done. ß-2 microglobulin
expression was determined as an internal reference.
Stimulus c-fos c-jun ß-2 microglobulin
-----------------------------------------------------
control 0 NC NC 1.05
control 6 NC NC 1.15
EGF 1.90 2.25 0.95
PMA 1.35 2.05 0.95
A23187 1.05 0.95 1.05
forskolin 0.95 ND ND
The actin microfilament system
The former
observations indicate that specific signalling pathways are
affected by µg. This could mean that µg affects a
limited number of gravity-sensitive cellular targets. The actin
microfilament system is a possible candidate for mediating
gravity effects in A431 cells. This assumption is based on EGF-
induced cell rounding in A431 cells, a process dependent on actin
polymerisation, being enhanced in simulated µg.8
Preliminary data obtained during the MASER 5-6 flights indicate
that µg causes increased actin polymerisation. The precise
role of actin as a gravity-sensitive cellular component has,
however, still to be established.
Acknowledgements
The authors would like to thank ESA for providing the CIS
facility, everyone involved in the successful MASER 3-6 flights
and SRON for financial support.
References
-
Gmünder, F. K. & Cogoli, A. (1988). Cultivation of single
cells in space. Appl. Microgravity Techn. I, 115-122.
-
Rijken, P. J., Boonstra, J., Verkleij, A. J. & de Laat, S. W.
(1994). Effects of gravity on the cellular response to epidermal
growth factor. Adv. Space Biol. Med. 4, 159-188.
-
Boonstra, J., Rijken, P. J., Humbel, B., Cremers, F., Verkleij,
A. J. & van Bergen en Henegouwen, P. M. P. (1995). The epidermal
growth factor. Cell Biol. Int. 19, 413-430.
-
Boonstra, J., Mummery, C. L., van der Saag, P. T. & de Laat, S.
W. (1985). Two receptor classes for epidermal growth factor on
pheochromocytoma cells, distinguishable by temperature, lectins
and tumor promoters. J. Cell. Physiol. 123, 347-352.
-
van Belzen, N., Rijken, P. J., Hage, W. J., de Laat, S. W.,
Verkleij, A. J. & Boonstra, J. (1988). Direct visualization and
quantitative analysis of epidermal growth factor induced receptor
clustering. J. Cell. Physiol. 134, 413-420.
-
Rijken, P. J., de Groot, R. P., van Belzen, N., de Laat, S. W.,
Boonstra, J. & Verkleij, A. J. (1993). Inhibition of EGF-induced
signal transduction by microgravity is independent of EGF
receptor redistribution in the plasma membrane of human A431
cells. Exp. Cell Res. 201, 373-377.
-
Rijken, P. J., Hage, W. J., van Bergen en Henegouwen, P. M. P.,
Verkleij, A. J. & Boonstra, J. (1991). EGF induces rapid
reorganization of the actin microfilament system in A431 cells.
J. Cell Sci. 100, 491-499.
-
Rijken, P. J., de Groot, R. P., Briegleb, W., Kruijer, W.,
Verkleij, A. J., Boonstra, J. & de Laat, S. W. (1991). Epidermal
growth factor-induced cell rounding is sensitive to simulated
microgravity. Aviat. Space Env. Med. 62, 32-36.
-
de Groot, R. P., Rijken, P. J., den Hertog, J., Boonstra, J.,
Verkleij, A. J., de Laat, S. W. & Kruijer, W. (1990).
Microgravity decreases c-fos induction and SRE activity. J.
Cell Sci. 97, 33-38.
-
de Groot, R. P., Rijken, P. J., den Hertog, J., Boonstra, J.,
Verkleij, A.J., de Laat, S. W. & Kruijer, W. (1991). Nuclear
responses to protein kinase C signal transduction are sensitive
to gravity changes. Exp. Cell Res. 197, 87-90.
About|
Search|
Feedback



 SP1206
SP1206
Published April 1997.
Developed by ESA-ESRIN ID/D.

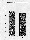



 SP1206
SP1206