
Sperm Motility under Weightlessness
Texus Flights 19 & 26
U. Engelmann
Medical Consulting, Sebastian-Bauer-Strasse 37,
D-81737 Munich, Germany
F. Krassnigg
Austrian Federal Environmental Agency,
Franz-Josef-Kai 1, A-5020 Salzburg, Austria
W.-B. Schill
Department of Dermatology and
Andrology, Justus Liebig University Giessen, Gaffkystrasse 14,
D-35392 Giessen, Germany
The aim of this study was to discover the motility
differences of frozen and thawed bull spermatozoa under
weightless and ground conditions. Significant differences were
found using a computerised sperm motility analyser. The analysis
showed alterations in straight line and curvilinear velocity, as
well as in linearity values. The amount of progressively motile
spermatozoa, including all spermatozoa with velocities >20
µm/s, increased significantly from 24±9.5% in the
reference test, to 49±7.6% in the µg test. In
conclusion, there is strong evidence that gravity influences
sperm motility.
Introduction
Several experiments and studies have shown that some processes
occur differently and more favourably under µg than under
terrestrial conditions. Consequently, certain properties can be
produced only under µg.
In this study, the motility of frozen and thawed bull
spermatozoa was investigated under weightlessness and compared
with motility under normal conditions on Earth. The influence of
gravity on functional processes of higher biological systems was
thus studied. For many reasons, the spermatozoon is an ideal
model to examine physiological functions, e.g. the study of
motility. It remains unknown to what extent and manner biological
functional processes such as cell differentiation, transportation
and development are influenced by gravity. Investigations
performed under µg should therefore contribute to the
understanding of the genesis, structure and function of gravity-
perceiving cells, and should elucidate the importance of gravity
on molecular mechanisms involved in basic biological functions.
The effect of gravity on sperm movement characteristics was
clearly demonstrated in this investigation.
The great interest in recent years in the objective analysis
of sperm motility has made it increasingly apparent that
progressively motile spermatozoa and their movement
characteristics are of biological and, hence, clinical
importance.
Materials and methods
Material
Semen samples were obtained from
bulls at the Grub animal breeding and insemination station in
Germany, collected by an artificial vagina. Samples showing at
least 80% sperm motility were diluted to a concentration of 40-
50x106/ml and frozen in 0.5 ml straws according to the
method described in ref. 1. The frozen samples were thawed and
maintained at 39°C until examination.
Experimental design and mission
performance
The experiments were carried out in
November 1988 (TEXUS 19) and May 1990 (TEXUS 26). The samples
were measured in a specially developed airtight and constant-
temperature (39°C) chamber (Strömberg-Mika, Bad
Feilnbach, Germany), as part of a TEXUS module. The thawed
spermatozoa were observed under a negative phase contrast
microscope (Nikon ELWD 20x objective; Nikon CFW 15x ocular). The
samples were inserted into the payload about 40 min before
launch. After lift-off, the semen samples in the chamber could
be monitored directly online with a TV camera. The experimenter
on the ground was able to influence and control experiment
parameters via telecommand channels to maintain focusing
sharpness. Another channel allowed 12 (TEXUS 19) and 17 (TEXUS
26) different fields of vision during the µg phase. The
images were recorded on the ground.
The video tapes were evaluated by a computerised semen
motility analyser (Ibac-Cell, AT-CAS 188, Brunner, Munich,
Germany), which allowed the measurement of total and progressive
sperm motility as well as determination of sperm
velocity.2-5 The frame rate for motility
analysis was 25/s, the time between successive frames was 40 ms,
and the actual observation time for each spermatozoon was 600 ms.
Only those cells that could be detected in eight successive
frames were evaluated. Curvilinear velocity was calculated from
the sum of the straight line distances between all points along
the track. Straight line velocity was calculated from the
straight line distance between the track's first and last points.
In addition, within the group of progressively motile
spermatozoa, three classifications could be distinguished,
characterising the quality of motion6 by calculation
of the linearity values (ratio of straight line to curvilinear,
S/V; Fig. 1).
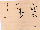
Fig. 1. Examples of motion patterns of spermatozoa. Motility
characteristics derived from video acquisitions and processing
of the sperm track. V = curvilinear velocity (µm/s); S =
straight line velocity (µm/s), showing different motion
patterns within progressively motile spermatozoa. Progressively
motile spermatozoa are divided into three groups according to
their S/V ratios: 0.9-1.0 (linear forward); 0.8-0.9 (not linear
forward); 0.0-0.8 (other movement pattern).
The motility of another aliquot of the same ejaculate was
examined under identical conditions on the ground to compare with
the TEXUS 19 data. For TEXUS 26, the reference test was carried
out immediately before launch using the same sample. The
advantage of using the same sample was the elimination of
variations within the measurements normally found in different
aliquots of the same ejaculate.
Totals of 761 and 698 spermatozoa were evaluated under 1 g and
0 g, respectively, on TEXUS 19. By increasing the fields of
vision, 1154 and 1090 could be observed during TEXUS 26. The
Wilcoxon signed rank test was used for analysis of significance.
Results
In TEXUS 19, no differences between quantitative sperm
motility (which refers to velocity) under weightlessness and
under gravitational conditions on Earth were detectable. Total
motility and the percentage of spermatozoa with progressive
motility (velocity >20 µm/s) were nearly identical under 1
g and µg conditions (Fig. 2). However, computer analyses
showed significant alterations in motility pattern, i.e. changes
in the path shape, expressed as the linearity value. The number
of linear forward motile spermatozoa (linearity value >.9) rose
significantly (Fig. 3) from 42±9% in the ground reference
test to 73±8% in the µg test (p<.005).
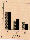
Fig. 2. Comparison of quantitative motility on the ground (1
g) and under weightlessness (µg) in TEXUS 19. Spermatozoa
are classified into three groups according to their velocity
values. a: total motility; all spermatozoa that move >10
µm/s. b: progressive motility; all spermatozoa that move
forward at >20 µm/s. c: local motility; all spermatozoa that
move at 10-20 µm/s. n.s. = not significant.
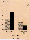
Fig. 3. Moving patterns of progessively motile spermatozoa on
the ground compared with those under weightlessness in TEXUS 19.
The path shape is characterised and expressed by the S/V ratios
(= linearity values). These are defined as in Fig. 1.
On the other hand, there were no significant differences in
straight line and curvilinear velocities. Velocity values showed
only small deviations for both samples under weightlessness and
the reference on Earth (Table 1). However, these small
differences are sufficient to shift the S/V ratio so that the
motion of linearly moving spermatozoa rose significantly. For
TEXUS 26, total motility was 71.4±7.2% in the reference test
and 79±6.2% under µg conditions (p<.005). A highly
significant statistical difference could be observed in the group
of progressively motile spermatozoa (Fig. 4). The portion of
progressive motility increased from 23.9±9.5% under ground
conditions to 49±7.6% (p<.005) during the flight.
Curvilinear velocity values within this group also rose
significantly from 28.8±2.5 µm/s on Earth to
31.9±3.2 µm/s in space (p<.01; Table 1). At the same
time, local motility decreased from 47.5±9.1% in the
reference test to 30±6.5% in the µg test (p<.005; Fig.
4). This implies that 17.5% of the local motile spermatozoa
(defined as cells with velocities of 10-20 µm/s) could
overcome the theoretically defined velocity borderline of 20
µm/s. Under the influence of weightlessness, these cells
could be classified by computer analysis as progressively motile
spermatozoa. Regarding qualitative motility patterns (path
shape), the portion of linear forward motile spermatozoa with
linearity values >.9 rose significantly from 30.5±12.8% on
the ground to 42.2±16.1% (p<.01) during the flight (Fig. 5).
This means an increase in linearity of 12% under the
influence of weightlessness.
Table 1. Velocity data of bull spermatozoa on Earth and under weightlessness.
S=Straight line velocity (µm/s). V = curvilinear velocity (µm/s).
TEXUS 19 TEXUS 26
µg 1 g µg 1 g
-------------------------------------------------------------------------------
Velocity (S) 29.1±9.4 27.5±6.2 26.9±2.8 23.8±2.5*
Velocity (V) 29.8±9.9 30.9±7.5 31.9±3.2 28.8±2.5**
--------------------------------------------------------------------------------
*p<0.005 **p<0.01
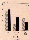
Fig. 4. Comparison of quantitative motility on the ground (1
g) and under weightlessness (µg) in TEXUS 26. Spermatozoa
are classified into three groups according to their velocity
values. a: total motility; all spermatozoa that move >10
µm/s. b: progressive motility; all spermatozoa that move
forward at >20 µm/s. c: local motility; all spermatozoa that
move at 10-20 µm/s.
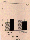
Fig. 5. Motion patterns of progessively motile spermatozoa on
the ground compared with those under weightlessness in TEXUS 26.
The path shape is characterised and expressed by the S/V ratios
(= linearity values). These are defined as in Fig. 1. n.s. = not
significant.
Discussion
Using bull spermatozoa, it was possible for the first time to
show the influence of gravity on the motility of biological
systems. The first indications of µg effects were detectable
in the TEXUS 19 experiment, and could be confirmed after reflight
on TEXUS 26. The differences in results between the two flights
were a consequence of the improved experimental conditions on the
second mission. The use of the same ejaculate sample for both the
reference and µg tests was highly beneficial. On TEXUS 19
the reference test was carried out by thawing another sample of
the same ejaculate.
The detected changes in motility characteristics are
definitely due to weightlessness, as the results of the
experiments demonstrate. Additional proof was provided by a long
series of supporting ground experiments in which launch
conditions such as acceleration (12 g) and vibration were
simulated. No changes in sperm movement properties were
detectable in any case. Microgravity conditions thus favour the
motility pattern of spermatozoa and might possibly improve
fertilisation capacity. On the other hand, there is still a wide
range of explanations for these clear effects.
Possibly the enhancement is due to changes in membrane
permeabilities and/or energy balances. It might be that, under
µg, energy-supplying mechanisms are more economical and more
rapid. The activity of membrane-bound enzymes such as thymidine
kinase (E. C. 2.7.1.21) might be influenced by µg-induced
changes of the membrane permeability, affecting DNA
synthesis.7
Results from other space missions substantiate the above
assumptions, proving that weightlessness can have an influence
at cellular level. The Franco-Soviet Salyut 6 Cytos experiments
on a unicellular organism, the Paramecium, showed a strong
stimulation of the cell growth rate.8 An increase in
cytoplasmic hydration, a drop in total protein content and a
change in the electrolytic content were found, among other
effects. This was particularly shown by a decrease in
intracellular calcium, probably related to structural changes in
the cytoskeleton proteins, especially in their sites for calcium
binding, or to modifications of the energetic metabolism
connected with ciliary movement.9
Ref. 10 reported that embryonal lung cells (WI-38) cultivated
under µg conditions showed a 20% reduction in glucose
consumption compared to ground-based experiments. This suggests
that energy consumption was lower for cells cultured in the
absence of gravity. On the basis of these findings and the
results from the TEXUS experiments, it would be of great interest
to extend these studies to other biological systems, especially
to primate cells.
Acknowledgements
This work was supported by grants from the German Federal
Ministry of Research and Technology (contract number QV 87010).
The authors would like to thank Dr O. Haeger, Test and
Insemination Station Grub, Germany, for providing bull
spermatozoa, and Mrs A. Full for her expert technical assistance.
References
- Steinbach, I. & Foote, R. H. (1967). Osmotic pressure and pH
effects on survival of frozen bovine spermatozoa. J. Dairy
Sci. 50, 205.
- Katz, D. F., Davis, R. O., Delandmeter, B. A. &
Overstreet, J. W. (1985). Real-time analysis of sperm motion
using automatic video image digitization. Comput. Methods
Programs Biomed. 21, 173.
- Katz, D. F. & Davis, R. O. (1987). Automatic analysis of
human sperm motion. J. Androl. 8, 170.
- Knuth, U. A., Yeung, C.-H. & Nieschlag, E. (1987).
Computerized semen analysis: objective measurement of semen
characteristics is biased by subjective parameter setting.
Fertil. Steril. 48, 118.
- Mortimer, D., Serres, C., Mortimer, S. T. & Jouannet, P.
(1988). Influence of Image Sampling Frequency on the Perceived
Movement Characteristics of Progressively Motile Human
Spermatozoa. Gamete Res.20, 313.
- Auger, J. & Dadoune, J. P. (1988). Computerized sperm
motility and application of sperm cryopreservation. Arch.
Androl. 20, 103.
- Tairbekov, M., Voronkov, L. A. & Gzova, A. (1982). Some
physical and biochemical features of cells of carrot gall
developed in weightlessness. Space Biology and Aerospace
Med. 16, 62.
- Planel, H., Tixador, R., Nefedov, Y., Gretchko, G.,
Richoilley, G., Bassleer, R. & Monrozies, E. (1981). Space flight
effects on Paramecium tetraaurelia flown aboard Salyut 6 in Cytos
1 and Cytos M experiments. Adv. Space Res. 1, 95.
- Tixador, R., Richoilley, G., Templier, J., Monrozies, E.,
Maotti, M. & Planel, H. (1981). Etudes de la teneur intra et
extracellulaire des electrolytes dans les cultures de paramecies
realises pendant un vol spatial. Biochim. Biophys. Acta
649, 175.
- Montgomery Jr., P. O. B., Cook, J. E., Reynolds, R. C.,
Paul, J. S., Hayflick, L., Stock, D., Shults, W. W., Kimsey, S.,
Therolf, R. G., Rogers, T. & Cambell, D. (1978). The response of
single human cells to zero gravity. In vitro 14, 165.
About|
Search|
Feedback



 SP1206
SP1206
Published April 1997.
Developed by ESA-ESRIN ID/D.
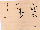

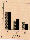
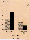
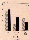



 SP1206
SP1206