
Fertilisation and Development of Xenopus Eggs on Sounding Rockets
and in a Clinostat
G. A. Ubbels
Netherlands Institute for Developmental
Biology, Hubrecht Laboratory, Uppsalalaan 8, Utrecht, The
Netherlands
Introduction
Living organisms have developed special structures by
evolution to perceive and cope with the gravity vector. In
various organisms, the absence of gravity perturbs particular
cell functions essential for normal embryonic development, such
as cell proliferation and adhesion. Since classical embryological
experiments1, 16, 17, 23
suggested that gravity is involved in determining the amphibian
embryo's spatial structure, gravity may act as a signal in
certain developmental processes, as do, for example, heat shocks
at particular times in development. Microgravity shocks or longer
periods of different gravity loads may influence normal
development through, for example, perturbation of embryonic axis
formation.
In the radially-symmetrical mature egg of Xenopus laevis
the polar 'animal-vegetal' axis roughly foreshadows the
embryo's main body axis. However, the egg needs some cue(s) for
establishing additional polarities, prerequisite for normal
development. As in many species, the sperm acts as such an
additional cue in the, normally, monospermically-fertilised
Xenopus egg.2, 23
Pigment concentrating around the Sperm Entry Point (SEP) marks
the meridian that foreshadows the embryo's prospective ventral
side. Since, in the majority of eggs, the blastopore forms at the
meridian about 180° away from the SEP, the embryo's general
body pattern is established from that time on. However, the
dorso-anterior and ventro-posterior polarities can still be
altered, by the influence of gravity and centrifugal forces,
which cause the rearrangement of yolk
components.2, 3, 7, 8, 23
This suggested that gravity in conjunction with the sperm
establishes the dorso-ventral polarity. It was logical to test
this hypothesis by experiments under µg during Spacelab
flights 14, 16, 24, 25
and Sounding Rocket (SR)
missions.4, 15, 16 for
rev., 18-22, 26
Materials and methods
Biological materials
Eggs of the South
African clawed toad, the anuran amphibian Xenopus laevis,
are favourite subjects for developmental biologists. They are
relatively large (~1.2 mm), easy to obtain and, because of their
size, fairly easy to manipulate. As a result, much detailed
information about the development is available (see refs. 6, 12,
16 for reviews). Over the years at the Hubrecht Laboratory,
suitable female toads have been pre-selected and skin-
marked.27 They could be relied on to provide
sufficient numbers of eggs at predictable times and the eggs
could survive the disturbances of launch and reentry, even after
24 hr of storage under Shuttle experiment preparation and launch
conditions.14, 17, 24, 25
About four weeks before arrival of the toads at ESRANGE in
Sweden, we set up a small amphibian facility with a simple water
pumping system, thus filtering the circulating tap water and
maintaining the temperature at around 22°C. The stress-
sensitive toads were best transported packed in cooled insulated
picnic boxes, partly filled with wet foam cuttings,24, 25
tranquillising them during the flight. Special care
has to be taken to avoid mechanical shocks and large temperature
variations. For the TEXUS 17 and MASER 3 missions, a private
plane was hired to ensure the toads' safe arrival. Transportation
to Kiruna by a commercial flight (as was done for MASER 6) is
risky, particularly as the toads have to be transferred twice to
another plane. Immediately on arrival, about three weeks before
launch, about 30 Xenopus laevis females and about 40 males
were placed separately into the aquaria. About a week later, the
animals were rather well acclimatised and produced healthy eggs
after hormonal stimulation under standard conditions of the
Hubrecht Laboratory.
The eggs' restricted viability, the relatively long 'late
access' period and the shortage of Payload Specialist time,
especially at the beginning of Space Shuttle missions, prompted
us to design an Automated Experiment Container (AEC) for the
experiment on Spacelab IML-1.14, 26 This
proved to be a real advantage when SR flight opportunities became
available regularly. Hardware and live materials should be able
to handle short periods of vibration, acceleration and
deceleration that are intensified compared to those during
Shuttle launches, but the much shorter late access period is an
important advantage for performing biological experiments in
µg. Unfortunately, as with Shuttle launches, there is the
increasing tendency for lengthening the late access period for
SR launches (Fig. 1).
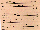
Fig. 1. Summary of experiments with Xenopus eggs in simulated
and real microgravity. Line 1. Upon sperm penetration, the egg
rotates as an entity in its capsule, turning its an-veg axis
upright the yolky, heavy half down and the pigmented animal
hemisphere up. Line 2. Experiments on clinostats were made after
artificial fertilisation on the bench, or (Line 3) by
fertilisation in the clinostat. Line 4. The experiments in real
µg (~6 min), as on TEXUS 17, are handed over (HO) relatively
shortly (1-2 hr) before Launch, though weather conditions or
experiment malfunction can cause a delay (MASER 3, Line 5) or
launch abort (as happened 6 days running with TEXUS 17). Line 6.
MASER 6's eggs were fertilised under µg in flight; the first
development of eggs was Line 7. Video-recorded shortly after
fertilisation on Earth at L-5 min 30 s, from L-4 min to L-1 min,
during L+73 s to L+419 s (the µg period) and after landing,
15-21 min after launch. Line 8. Fertilisation in a flight 1
g-centrifuge, simultaneously with fertilisation in µg,
served as the proper control to discriminate whether any
developmental anomaly found was due to a real µg effect. In
addition, control eggs were fertilised after landing (see Fig.
5). Lines 9/10. Experiments in real µg on a Space Shuttle
mission have to be delivered much earlier than for sounding
rockets. For IML-1 and IML-2 it approached 15 hr before launch,
and the gap is expected to become longer. Launch delays and
aborts may also occur. Fertilisation in the Shuttle could be
performed only about 7 hr after reaching µg, i.e. 24 hr
after stripping eggs from the females (hardware loading
inclusive).
Xenopus males and females were hormonally stimulated,
precisely timed before a
mission.15, 18, 19 The males were
killed for removal of the testes and eggs were stripped from the
females. Immediately after stripping, eggs from two of the
usually four injected females were individually selected. Within
2 hr, the AECs were each loaded with salt solutions, histological
fixative, one testis and two groups of 15 selected eggs from two
different females. After stripping the same females for a second
time, a second set of AECs was prepared for Earth control, to be
activated 2 hr later. Selected eggs were also fertilised in some
laboratory modules and dishes on the bench.
Hardware
The AEC (Fig. 2.1, Table 1)
comprises a perspex cylinder block (79.5x19.0x33.1 mm, height
with electronics 40 mm) equipped with spring-activated pistons
for sequential operation, governed by a
microprocessor.14, 16, 18, 26
In Shuttle flight experiments, each AEC was contained
individually in a Biorack Type-IE container (Figs. 2.1 and 4)
equipped with a connector for electrical activation and recording
the temperature and housekeeping signals.
Table 1. Activation programmes for the Automated Experiment
Containers on MASER 6 (time in seconds).
------------------------------------------------------------
Onset 10 s after yo-yo despin
T1 A1 R1 T2 A2 R2
P1 0 30 210 0 30 210
P2 (*) 0 30 210
T A R M F1 F2
P3 0 30 60 170 210 -
------------------------------------------------------------
Onset 5-7 min before launch
T1 A1 R1 T2 A2 R2
V 0 30 210 0 30 210
------------------------------------------------------------
F2 contained additional eggs and testis to perform manual
fertilisation after landing. *fertilisation on Earth (also see
Fig. 5)

Fig. 2. Fertilisation of Xenopus Eggs: some Automated
Experiment Containers (AECs). Fig. 2.1. AEC used on TEXUS 17,
MASER 3 and Space Shuttle IML-1. 20, 24, 25
It consists of a perspex cylinder block
(79.5x19.0x33.1 mm; 40 mm with electronics) equipped with spring-
activated pistons governed by a microprocessor for sequential
operation, providing fluid transfers between the different
compartments of the closed system.18, 20, 26
Eggs were stripped from the females and selected. Within 2 hr,
the AECs were loaded with salt solutions, histological fixative,
a testis and 2x15 selected eggs. After another 2 hr and stripping
the females for a second time, a second set of AECs was prepared
for activation on Earth as a control; control eggs were also
fertilised in some laboratory modules and in dishes on the bench.
E: egg cylinder, and T: testis cylinder, both with 100% MMR and
2x15 eggs or one testis. A and R: cylinders with 10% MMR for
fertilisation; M with 25% MMR for embryo culture; G with extra-
contained glutaraldehyde fixative. Fig. 2.2.1. Modified AEC
(mAEC) for the IML-2 Shuttle mission, providing improved
circulation of fluids in the block, excellent visibility of two
groups of embryos from different females for video-recording, in
the now laterally positioned egg compartment (with two sub-
divisions), and the possibility for an extra wash or post-
fixation.4 Fig. 2.2.2. mAEC back view. Plungers,
programmed for sequential activation to perform fertilisation are
down (T, A, R), the additional three provide washing and the two
histological fixations (M, F1, F2; not yet
activated).4
Fig. 2.3. mAEC used on MASER 6, with two lateral egg compartments
and two sets of plungers, independently programmable for
fertilisation. Twice as many eggs could be fertilised in one go.
However, histological fixation could not be performed in this
module.4 The illustration shows how the left egg
compartment is adapted for videorecording. The various inserts
are interchangeable. 15 eggs from each of two different females
and a testis were stored separately in the AECs, in 100% MMR (230
m Osmol and pH 7.8). The sounding rocket storage and
fertilisation temperature was 18°C. Shuttle experiment
storage was at 10°C; fertilisation in 10% MMR and culture
at 22°C in 25% MMR. Eggs from only one female were available
on MASER 6.
2.2.1 The 'Frog Eggs' experiment on TEXUS 17
In the 'Frog Eggs' experiment on TEXUS 17, eight AECs, each
with its own temperature sensor, were housed together but
individually electrically-activated in an air-tight double-walled
aluminium box (Fig. 3.1-
3.3)15, 16, 18, 19, 26
specially designed and built by CCM (Nuenen, The
Netherlands) as a subcontractor to ERNO (Bremen, Germany). The
box provided excellent insulation for the flight duration and the
conditions; the experiment units' internal temperature rose by
only 1°C both in flight and on Earth; the pressure remained
constant at 1 bar.15, 16, 26It was
loaded aboard only 45 min before launch, inserted into TEM 06-15
(Fig. 3.4), a modification of TEM 06-11.21The
autonomous power supply and the electronics package for recording
housekeeping data, temperature, etc were mounted under the
experiment platform. The AEC electronics and all the plungers
(modified after the Spacelab D1 Shuttle mission) functioned
flawlessly.
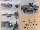
Fig. 3. TEXUS module TEM 06-15 used for the fertilisation of
Xenopus laevis eggs. Fig. 3.1. Air-tight double-walled aluminium
box (120x150x350 mm). Fig. 3.2-3.4. 2x2 AECs were positioned
along a horizontal bar plugged in on both sides of a midway
vertical panel. The panel carried electrical connections on both
sides for power supply and data recording (housekeeping signals
and egg cylinders temperatures) from the eight experiment
modules, which were individually activated. The autonomous power
supply and the data-recording electronics package were positioned
under the circular experiment platform (Fig. 3.4). Fig. 3.5. TEM
06-15 flew for a second time with Xenopus eggs on MASER 3. This
time, the electronic unit was positioned in the box's front part.
2.2.2 The 'Frog Eggs' experiment on MASER 3
As on
TEXUS 17, eight AECs were successfully flown in TEM 06-15, but
the electronics serving these modules were centralised in the
front part of the box 26 (Figs. 3.5 and 4), making the
AECs cheaper and reusable. The mission was another successful
flight test for the AEC.
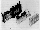
Fig. 4. Automated Experiment Container (AEC). See ref. 25.
Fig. 4.1. AEC partially inserted into the Biorack Type-IE
container, with incubator-A connector (c). Fig. 4.2. AEC without
Type-IE container, as used on TEXUS 17 (see Fig. 2.1). Ct: wires
connecting the microprocessor with the panel in the centre of the
experiment box. Fig. 4.3. Mannually-operated flight-type
laboratory module, without electronics. The plungers can be
operated by turning the screws (s) on top.
2.2.3 The 'Frog Eggs' experiment on MASER 6
This
time, the automated hardware was flown in Fokker Space's CIS-4
module (see Figs. 3.9, 3.12.c. and 3.13.a in Section 3 of this
volume) and activated in two separate (0 g and 1 g) boxes. As on
MASER 3, 26 the electronics serving the AECs was
centralised. We used a modified version of the AEC (further
denoted as mAEC 4 ;
Figs. 2.2-2.3), which now contained two sets of three cylindrical
holes, each set with an additional lateral egg compartment. The
two sets were operated independently.
Thus fertilisation in each set could be performed at
separately programmable times and twice as many embryos could be
fertilised in one unit (Figs. 2 and 5). Furthermore, the modified
arrangement of the different compartments improved the
circulation of the various fluids in the mAECs and the visibility
of the developing embryos in the units was excellent for video-
recording (Fig. 2.3). However, histological fixation could not
be performed in these modules.
In the mAEC's IML-2 version (Figs. 2.2.1. and 2.2.2)4
there was only one egg compartment and two of the six
cylindrical compartments were available for histological
fixatives. During the MASER 6 flight, two such modules were
activated for an in-flight functional hardware test, including
histological fixations. Both mAEC configurations performed well
(Fig. 5).
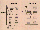
Fig. 5. Schematic overview of all MASER 6 experiment
containers (with the mean of fertilisation rates per group of 15
eggs) fertilised in flight, either in µg (92%) or in the
flying 1 g centrifuge (88%), and on Earth (72%). This time, eggs
were used from one female because only one of the injected
females produced good quality eggs, in a number just large enough
to load 2x3 double and two single (IML-2) mAECs for flight and
1x3 double and one single mAEC for the ground control. Two mAECs
were first loaded for video-recording in µg and on Earth,
with eggs from the first strip. The ground control mAECs were
activated 2 hr later than those in the two flight boxes. Because
of poor handling during the air transfer, many of the pre-
selected frogs died on the way to Kiruna and not many were left
by the time of the fourth launch attempt. All mAECs therefore had
to be loaded with eggs from only one female, and hardly enough
good quality eggs were left for the ground controls, which is
reflected in the total fertilisation scores. Moreover, one of the
plungers failed in a ground mAEC, which is known to reduce the
fertilisation rate. Only one of the cylindrical holes in the
single mAECs (see Figs. 2.2.1 and 2.2.2) contained fixative. In
the sixth cylinder there were full-strength MMR and 15 eggs and
a testis, each in a separate membrane. Back in the laboratory,
immediately after return from flight, the different groups of
these eggs were fertilised manually with sperm flown in the same
module. As an additional control, automated fertilisation was
performed immediately after landing. In µg flight and in the
ground control box (see Fig. 6), the cortical contraction was
video-recorded in some of the eggs in one half of a double mAEC,
which was provided with a special insert (Fig. 2.3) to keep the
eggs in focus. As a control, the eggs in the other half of these
mAECs were fertilised simultaneously with the recorded ones.
Fertilisation rate variations could also be caused by differences
in sperm fertility in the different testes.
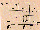
Fig. 6. Video-recording of the development of Xenopus eggs in
µg. After fertilisation at L-5 min 30 s, recordings were
made at L-4 min to L-1 min, in µg from L+75 s to L+419 s and
after landing from L+15 min to L+21 min. In flight, n=3; on
Earth, n=5. The figure shows only a very small temperature
change, which cannot explain the very limited, but significantly
earlier, start of the cortical contraction in flight (585 10 s
after in-flight fertilisation, versus 679 9 s on Earth). The very
small number of eggs recorded and the possible influence of
launch disturbances prevent a firm conclusion. The minor, though
significant, acceleration under µg of the cortical
contraction initiation time prompts the suggestion of a reflight
on MAXUS.
A 1g control centrifuge and a video recorder were used for the
first time on MASER 6. Fertilisation was performed simultaneously
under µg and 1 g conditions, so that flight perturbations
other than varying g-loads were identical for all egg samples.
Apart from the 1 g flight centrifuge, 1 g control embryos were
obtained by activating a similar set of mAECs on Earth (Fig. 5).
In both cases, the cortical contraction of fertilised eggs was
recorded in the specially-designed facility; the eggs in the mAEC
were held in focus with a special insert 4 (Figs.
2.4). Both of these newly-designed facilities, the flight
centrifuge and the video recorder, functioned nominally.
After fertilisation of the different experimental groups in
the mAECs activated on MASER 6 (Fig. 5) or after fertilisation
in a fast-rotating clinostat (60 rpm), the embryos were carefully
grown on Earth after retrieval and subsequently histologically
fixed at different times. After staining the nuclei in the fixed
blastulae, in optical serial sections of whole mounts analyzed
by Confocal Laser Scanning Microscopy (CLSM),5, 16
we determined the number of blastomeres at the 8th
cleavage and the shape and size of the blastocoel, using CLSM
software for planimetry. In situ hybridisation analysis of whole
mounts was applied for checking proper gastrulation in µg
and in control embryos.4 Only eggs from one female
were available for loading MASER 6's mAECs.
Biological results and discussion
'Frog Eggs' on TEXUS 17
Launched: 2
May 1988 (see references 15, 18-21, 16 for review) Crew: G. A.
Ubbels, Principal Investigator (PI); J. Narraway (Hubrecht Lab).
R. de Groot (Hubrecht Lab), H. van Soest (CCM); P. Kyr and P.
Kuker (MBB/ERNO) for mission support.
Objectives: test the experiment container's plunger
function under real flight conditions; determine whether
Xenopus eggs could be fertilised during the brief µg
period available (6-7 min); if so, whether under µg sperm
penetrated only the animal hemisphere and whether the polyspermy-
block, which at 1 g is caused by a transiently-enhanced egg
membrane potential, also functions in µg.
Results and discussion
The eggs of a
vertebrate, the anuran amphibian Xenopus laevis, were
successfully monospermically fertilised and histologically fixed
in µg for the first time. Analysis by normal histology and
Scanning Electron Microscopy (SEM) revealed that, as in normal
development, only one sperm penetrated somewhere in the egg's
pigmented animal half. The polyspermy-block thus functioned as
well in µg as under 1 g (Fig. 7). This was the first
developmental biological experiment ever performed on a sounding
rocket (SR). It demonstrated the feasibility of SR biological
experiments in spite of the relatively high levels of
disturbances (e.g. vibration, acceleration and deceleration)
during launch and re-entry. SRs were thus shown to be appropriate
vehicles for performing short-duration biological tests.

Fig. 7. The single sperm penetrating the pigmented animal cap.
6 µm histological paraffin section. Fixation 138 s after
fertilisation in the AEC during TEXUS 17. See ref. 18.
'Frog Eggs' on MASER 3
Launched: 10 April 1989 (see references 18, 20, 22, 26; 16
for review)
Crew: G. A. Ubbels, PI; J. Narraway and
S. Kerkvliet (Hubrecht Laboratory); H. van Soest (CCM) and Dr.
P. Kyr and P. Kuker (MBB/ERNO) for mission support.
Objectives: confirm the TEXUS 17 results, achieving
fertilisation and histological fixation in µg at slightly
different times; perform two pilot experiments: fertilise and fix
eggs in an AEC that were first soaked in Hoechst 33258 (see
below), and fertilise eggs in an AEC for live retrieval and
careful culture on Earth.
Results and discussion
The MASER 3 experiment
was a successful repeat of the TEXUS 17 experiment: sperm and
eggs fused in-flight with a high score, even within the first
minute.22 The eggs were successfully fertilised and
histologically fixed in flight in one of the eight AECs after
storage for almost 6 hr in Hoechst 33258. This is a rapidly
penetrating DNA-specific bisbenzimide fluorochrome, which raised
specific UV-fluorescence even after histological fixation. Both
UV-fluorescence and subsequent SEM analysis 18, 20, 22
confirmed that sperm-egg fusion in
µg starts real sperm penetration which, as shown later on
IML-1, does indeed initiate embryonic development. In a second
AEC, eggs again from two different females were fertilised but
not histologically fixed. The embryos survived re-entry. They all
developed normally but only until the gastrula stage before,
depending on the egg sample, dying in the period between
gastrulation and neurulation. Only four out of ten embryos from
one female continued to develop, and these developed similar
abnormal axes 18, 20, 22 (Fig. 8).
It was thus concluded that a µg shock during
fertilisation and/or initiation of development might perturb axis
formation. The proper control, i.e. an onboard 1 g centrifuge,
was not available at that time. However, a simulation experiment
in laboratory modules mounted on a bicycle wheel 9
showed that the morphogenetic abnormalities found after
fertilisation in the rocket were not due to variations in the g-
levels simulated in that experiment and applied in a pattern
identical to that on MASER 3.18, 20, 22
Vibrations were not included in this simulation.
Eggs fertilised in MASER 6's 1 g flight centrifuge (see below)
developed seemingly normally.4, 16


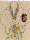
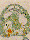
Fig. 8. Xenopus laevis embryos 5 days after fertilisation on
MASER 3 (7 min µg, retrieval within 15 min). The embryos
survived re-entry and gastrulated seemingly normally but,
compared with the controls, development was retarded. They were
slightly microcephalic and tail formation was strongly reduced.
Analysis of 6 µm histowax sections (fixation in Bouin
d'Hollande; alcoholic dehydration; azofuchsin, anilin blue,
orange G stain; see ref. 23), showed that (bottom section) two
notochords (1 and 2) had developed independently, the second
apparently being induced by a secondary inducing centre. In a
second larve (top section), the notochord (not shown) was split
and tapetum tissue (a characteristic for eye development) was
found in the bottom of the diencephalon (2; 1=normal eye). These
developmental abnormalities could be explained if fertilisation
in µg causes abnormal cell interactions later on, during
gastrulation.22

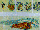
Fig. 9 Normally-developing embryos were retrieved from MASER
6, after fertilisation in µg and in the flying 1 g
centrifuge. We thus assume that formation of developmental
anomalies in the MASER 3 pilot experiment (see Fig. 8 and refs.
18 and 22) were due either to using a different launcher or the
inherent properties of egg samples produced by different females,
and/or the 2.5 hr launch delay (see Fig. 1). Fig. 9.1. Five day-
old abnormal Xenopus embryo from MASER 3 (see Fig. 8.) Fig.
9.2.1. Transverse 6 µm sections of a stage 42 12
Xenopus larve, grown on Earth after fertilisation on MASER
6 under µg, revealed only one normal, split notochord (n)
and many other signs of normal axis formation. Fig. 9.2.2. Serial
sagittal/longitudinal 6 µm sections through a larve from
another egg, fertilised in the flying 1 g centrifuge on MASER 6,
also showing normal morphology; for example, only one normal
notochord (n).
'Frog Eggs' on MASER 6
Launched: 4 November 1993 (see references 4,
5, 16 for review)
Crew: G. A. Ubbels, PI; M. Reijnen
and J. Narraway, (Hubrecht Laboratory); A. De Mazière
(SRON/NWO), J. Gonzalez-Jurado (ESA). J. P. Leysten and A. Koppen
(CCM) and Fokker Space for mission support.
Objectives: flight testing of design modifications of
the IML-2 experiment unit (mAEC); flight testing of the newly-
designed 1 g centrifuge and video-recording system; fertilisation
of Xenopus eggs in the mAECs simultaneously at 0 g and in
the 1 g centrifuge in flight; after retrieval and careful
culturing of the embryos on Earth, they were histologically fixed
at different developmental times. This could tell us whether a
µg shock indeed perturbs axis formation and/or causes other
developmental abnormalities (see the pilot experiment on MASER
3). Eggs were also fertilised and grown in a fast-rotating
clinostat (60 rpm). We determined the number of blastomeres and
the shape and size of the blastocoel, by using CLSM software for
planimetry, in optical serial sections from whole embryos, fixed
at the 8th cleavage, after staining the nuclei in whole
mounts.8 In situ hybridisation on whole mounts was
applied for checking proper gastrulation after fertilisation in
µg and 1 g, and subsequent culture and fixation on
Earth.4
Results and discussion
In the flight mAECs
activated during MASER 6, eggs from two pre-selected females were
fertilised at 0 g and in the 1 g centrifuge with equally high
(80-98%) fertilisation rates (Fig. 5). After retrieval, they were
grown on Earth and histologically fixed at different stages. The
analysis confirmed normal axis formation (Fig. 8d).
In the MASER 6 embryos fixed at the 8th cleavage stage and
after staining of the nuclei in whole mounts,5 we
determined the blastocoel volume, the thickness of the blastocoel
roof (in µm) in a 150 µm-diameter region around the
animal pole, and the number of cells in the blastocoel roof that
were hit by the animal/vegetal axis. These values did not
significantly differ in the flight-embryos between 0 g and 1 g
(Table 2). However, in the same embryos the ratio of the
blastocoel floor level to the total embryo height was
significantly different (Table 2 and Fig. 2 in ref.4). In embryos
from eggs fertilised at 0 g the floor was consistently closer to
the vegetal pole than in the embryos from eggs fertilised in the
flying 1 g centrifuge. This effect is independent of the µg
duration, as similar blastocoels were found in the embryos grown
and fixed during IML-2 after the 24 hr-delayed fertilisation
(Table 3). Nuclear counts in whole mounts of Xenopus
blastulae, grown after fertilisation and fixed at the 8th
cleavage on IML-2, also revealed no significant differences in
mitotic rates (Table 3). Together with previous data from
different cell systems, this suggests that, in a variety of cell
types, mechanisms regulating mitosis are different or differently
sensitive to µg.
Table 2. Analysis of blastulae grown after fertilisation at
the beginning of 6 min of µg on MASER 6.
--------------------------------------------------------------------------------------------------------------------------
Mean±SEM µg shock 1 g centrifuge P
Blastocoel volume (in nl) 41±8 45.0±:2.5 NS
(n=3) (n=4)
Blastocoel roof(thickness in µm) 258±12 247±13 NS
(n=4) (n=4)
Number of cells in blastocoel roof hit by A/V axis 4.00±0.18 3.45±0.29 NS
(n=4) (n=4)
Ratio of BC-floor level to embryo height 0.463±0.013 0.513±0.012 0.02<P<0.05
(n=4) (n=4)
--------------------------------------------------------------------------------------------------------------------------
NS = not significant. SEM = standard deviation of the mean
Table 3. Analysis of blastulae grown in continuous µg on IML-2.
Mean±SEM µg ground P
-----------------------------------------------------------------------------
Total number of nuclei/embryo 333±28 346±18 NS
(n=6) (n=6)
Blastocoel volume (in nl) 22±6 36±4 P<=0.05
(n=7) (n=9)
Total embryo volume (in nl) 610±14 580±11 NS
(n=7) (n=9)
-----------------------------------------------------------------------------
NS = not significant
After fertilisation in the rotating tubes of a fast-rotating
clinostat, i.e. in simulated µg, the embryos formed
consistently significantly smaller blastocoels with a thicker
roof than after fertilisation in the non-rotating tubes, i.e. at
1 g (Table 4). However, as after fertilisation in actual µg,
there was no general increase in cell number in the whole
blastula, nor an increase in the average cell number in the top
segment only (Table 4). In blastulae from freshly stripped eggs
fertilised in simulated µg, the blastocoel volume was
slightly, but significantly, smaller than in the 1 g controls.
At normal gravity, the blastocoel volume was not reduced by
delayed fertilisation. However, the blastocoel was much smaller
in simulated µg than in the 1 g controls, when eggs were
fertilised after 24 hr storage at 10°C, as is routinely done
in Shuttle experiments. Thus shorter as well as longer periods
of actual and simulated µg affect proper blastocoel
formation, probably through perturbation of fluid transport
between the blastocoel and its surrounding cells.16
A lower position of the blastocoel floor after clinostatting is
in agreement with results from previous experiments by others
10, 11, 28, 29 and,
from experiments in actual µg,13, 16
which supports clinostatting as a suitable method, though not a
replacement, for µg simulation experiments, even with the
relatively large Xenopus eggs.
Table 4. Blastulae developed in fast-rotating clinostat.
Mean±SEM µg ground P
---------------------------------------------------------------------------------------
Blastocoel volume (in nl) 24.8±2.4 33.3±2.2 0.01<P<0.02
(n=11) (n=12)
Blastocoel roof (thickness in µm) 204±6 152±6
(n=11) (n=6)
Cell number (whole embryo) 340±35 320±20 NS
(n=6) (n=11)
Cell number (top segment) 48±4 46±6 NS
(n=11) (n=6)
---------------------------------------------------------------------------------------
NS = not significant
Both the MASER 6 and clinostat experiments support the IML-2
results: the cleavage rhythm in Xenopus laevisis not
influenced by µg. Moreover, in real and simulated µg,
the blastocoel forms more centrally and vegetally, due to an
enlargement of the roof cells and not to a general increase in
cell numbers. In both clinostatted 10, 11, 16, 28, 29
and real-µg 4, 13, 16 embryos, these
morphogenetic differences disappear in the late blastulae or
early gastrulae. They do not interfere with mesoderm induction
and normal development.4, 13, 16
Xbra in situ hybridisation on whole MASER 6 embryos, after
fertilisation in flight at µg and 1 g, showed normal
gastrulation.4 As the size of the entire embryo was
unchanged, we assume that µg in some way interferes with the
regulation of fluid accumulation in the blastocoel, due to
changes in the osmoregulation governing the volume and
composition of the blastocoelic fluid (see ref. 16 and its quoted
references).
The cortical contraction,23 one of the first signs
of successful fertilisation, was analysed by video-recording
newly-fertilised eggs in flight and on Earth. After fertilisation
5 min 30 s before launch, the cortical contraction began in
flight slightly earlier than on Earth (Fig. 6). Sperm penetration
occurred on Earth in both groups, thus the differences in
initiation times originated within the few minutes thereafter,
and are probably not due to differences in the speed of sperm
penetration on Earth. On Earth, at the same temperature, the
entire process of contraction and relaxation takes about 12 min.
This preliminary experiment should be repeated and extended,
preferably on a longer flight such as MAXUS, so that a larger
number of eggs can be recorded simultaneously and video-recording
can be started in µg immediately after fertilisation (also
in µg).
To date, the available data from developmental biological
experiments on Earth and in space have shown that neither sperm
nor gravity are required for embryonic axis formation in
amphibians.16 Only Space Station experiments can tell
us whether gravity determines polarity(ies) in the ovarian egg
during maturation.
Acknowledgements
We gratefully acknowledge the skillful and enthousiastic
support of our collegues at ESRANGE, ESA and ESTEC, as well as
from MBB-ERNO during the TEXUS 17 and MASER 3 campaigns, and from
CCM and Fokker Space. We gratefully acknowledge the financial
support for the three sounding rocket experiments from ESA and
the Dutch Organisation for Scientific Research (NWO, now SLW)
through the Space Research Organisation of the Netherlands
(SRON/MG-013).
References
- Ancel, P. & Vintemberger, P. (1948). Recherches sur le
determinisme de la symetry bilaterale dans l'oeuf des Amphibiens.
Bull. Biol. Fr. Belg. (suppl) 31, 1-182.
-
Black, S. D. (1989). A step in embryonic axis specification in
Xenopus laevis is simulated by cytoplasmic displacements
elicited by gravity and centrifugal force. Adv. Space Res.
9, (11)159-(11)168.
- Black, S. D. & Gerhart, J. C. (1986). High frequency
twinning of Xenopus laevis embryos from eggs centrifuged
before first cleavage. Dev. Biol.116, 228-240.
- De Mazière, A., Gonzalez-Jurado, J., Reijnen, M. J.,
Narraway, J. & Ubbels, G. A. (1996). Transient effects of
microgravity on early embryos of Xenopus laevis. Adv. Space
Res. 17 (6/7)219-(6/7)223.
- De Mazière, A. M., Hage, W. J. & Ubbels, G. A.
(1996). A method for staining of cell nuclei, in Xenopus
laevis embryos, with cyanine dyes for whole mount confocal
laser scanning microscopy.J. Histoch. Cytoch. 44, 399-402.
- Elinson, R. P. & Holowacz, T. (1995). Specifying the
dorsoanterior axis in frogs: 70 years since Spemann and Mangold.
Curr. Top. Dev. Biol. 30, 253-285.
- Gerhart, J., Ubbels, G. A., Black, S., Hara, K. &
Kirschner, M. (1981). A reinvestigation of the role of the grey
crescent in axis formation in Xenopus laevis. Nature 292,
511-516.
- Kirschner, M. W., Gerhart, J. C., Hara, K. & Ubbels, G.
A. (1980). Initiation of the cell cycle and establishment of
bilateral symmetry in Xenopus eggs. Symp. Dev. Biol.
38, 187-215.
- Morgan, T. (1904). The dispensibility of the constant
action of gravity and of a centrifugal force in the development
of the toad's egg. Anat. Anz. 25, 94-96.
- Neff, A. W., Wakahara, M. & Malacinski, G. M. (1990).
Bifurcation of the embryo's axis: analysis of variation in
response to egg centrifugation. Int. J. Dev. Biol. 34,
391-398.
- Neff, A. W., Yokota, H., Chung, H. M., Wakahara, M. &
Malacinski, G. M. (1993). Early Amphibian (anuran) morphogenesis
is sensitive to novel gravitational fields. Dev. Biol.
155, 270-274.
- Nieuwkoop, P. D. & Faber, J. (1975). Normal Table of
Xenopus laevis (Daudin). 2nd ed. North-Holland Publ.,
Amsterdam.
- Souza, K. A., Black, S. D. & Wassersug, R. J. (1995).
Amphibian development in the virtual absence of gravity. Proc.
Natl. Acad. Sci. USA 92, 1975-1978.
- Ubbels, G. A. (1988). The role of gravity in the
establishment of the dorso ventral axis in the amphibian embryo.
In Biorack on Spacelab D1, ESA SP-1091. 147-155.
- Ubbels, G. A. (1988). A first fertilization of frog eggs
in space. Microgravity News from ESA 2, 19-23.
- Ubbels, G. A. (1997). Establishment of polarities in the
oocyte of Xenopus laevis. The provisional axial symmetry
of the full-grown oocyte of Xenopus laevis. Cellular and
Molecular Life Sciences 1, in Press.
- Ubbels, G. A. & Brom, T. G. (1984). Cytoskeleton and
Gravity at work in the establishment of Dorso-ventral polarity
in the egg ofXenopus laevis. Adv. Space Res. 4, (12)9-
(12)18.
- Ubbels, G. A, Berendsen, W., Kerkvliet, S. & Narraway, J.
(1989). The first seven minutes of a Xenopus egg
fertilized on a Sounding Rocket in Space. In Microgravity as
a tool in developmental biology. Special Symposium at the
11th International Congress of the International Society of
Developmental Biologists, ESA SP-1123, 49-58.
- Ubbels, G. A., Berendsen, W. & Narraway, J. M. (1989).
Fertilization of frog eggs on a Sounding Rocket in Space. Adv.
Sp. Res. 9, (11)187-(11)197.
- Ubbels, G. A., Berendsen, W., Kerkvliet, S. & Narraway,
J. (1990). Fertilization of Xenopus eggs in Space. In
Proc. 4th Symp. on Life Sciences Res. in Space. ESA SP-
307, 249-254.
- Ubbels, G. A., Kerkvliet, S. & Narraway, J. M. (1990).
Fertilization of Frog eggs in Space. In Texus 17-20
Abschluszbericht. 48-54.
- Ubbels, G. A., Berendsen, W., Kerkvliet, S. C. J. &
Narraway, J. M. (1992). Fertilization and Development of Eggs of
the South African Clawed Toad, Xenopus laevis, on Sounding
Rockets in Space. Adv. Space Res. 12, (1)181-(1)195.
- Ubbels, G. A., Hara, K., Koster, C. H. & Kirschner, M. W.
(1983). Evidence for a functional role of the cytoskeleton in
determination of the dorsoventral axis in Xenopus laevis
eggs. J. Embryol. Exp. Morph. 77, 15-37.
- Ubbels, G. A., Reijnen, M., Meijerink, J. & Narraway, J.
(1994). Xenopus laevis embryos can establish their spatial
bilateral symmetrical body pattern without gravity. Adv.
Space. Res. 14, (8)257-(8)269.
- Ubbels, G. A., Reijnen, M., Meijerink, J. & Narraway, J.
(1994). The role of gravity in the establishment of the dorso-
ventral axis in the amphibian embryo (05-NL-EGGS). In Biorack
on IML-1. ESA SP-1162, 175-185.
- Ubbels, G. A. & Willemsen, H. P. (1989). Automatic
experiment container (AEC) for fertilization of frog eggs in
Space. A multipurpose piece of classified Space hardware. In
Microgravity as a tool in developmental biology. Special
Symposium at the 11th International Congress of the International
Society of Developmental Biologists. ESA SP-1123, 75-78.
- Verhoeff-de Fremery, R. & Vervoordeldonk, F. J. M. (1982).
Skin autografts as markers in the toad Xenopus laevis.
Laboratory Animals 16, 156-158.
- Yokota, H., Neff, A. W. & Malacinski, G. M. (1992).
Altering the position of the first horizontal cleavage furrow of
the amphibian (Xenopus) egg reduces embryonic survival.
Int. J. Dev. Biol. 36, 527-535.
- Yokota, H., Neff, A. W. & Malacinski, G. M. (1993).
Analysis of experimental and quantitive parameters employed to
assess the effects of novel gravity forces on the amphibian egg.
Biol. Sci. Space 7, 85-100.
About|
Search|
Feedback



 SP1206
SP1206
Published April 1997.
Developed by ESA-ESRIN ID/D.
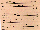


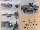
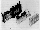
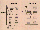
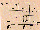



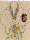
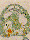

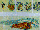



 SP1206
SP1206