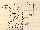
Figure 1. Layout of a typical FTIR system

A major concern during manned spaceflight is the quality of the cabin atmosphere. In particular, the crew could be endangered by the uncontrolled accumulation of gaseous trace contaminants arising, for example, from off-gassing (from structural materials, electronic equipment or materials used in experiments, etc.), from system failures (leaks, equipment over-heating, fires, etc.), or from the crew itself (metabolic products).
To ensure a safe atmosphere at all times, these trace gases need to be monitored and controlled so that they remain below certain safe limits. These limits are known as SMAC Spacecraft Maximum Allowable Concentration values and have been defined as a result of medical considerations, previous space-flight experience and analogous terrestrial experiences such as in submarines and during saturation diving. SMAC values vary considerably according to the chemical compound considered but, within a normal atmosphere, they are typically measured in parts per million (ppm) or even parts per billion (ppb). SMAC values also vary as a function of the mission's length (time of exposure), higher SMACs generally being tolerable during shorter missions. The ESA Atmosphere Quality Standard, PSS-03-401, contains a typical SMAC list, but these lists are continually being reviewed (extended and updated) in the light of new experience.
The strategy that has been adopted to date for the management of trace contaminants, for example in Spacelab, is basically to minimise the off-gassed products in the atmosphere by the careful selection and cleaning of materials, and to size the contamination control system with a sufficient margin to ensure that SMAC values will not be exceeded. The actual monitoring of trace gases is limited only to the most toxic or explosive ones such as carbon monoxide and hydrogen. The major atmospheric constituents, nitrogen, oxygen, water vapour and carbon dioxide, are not regarded as trace gases and are monitored by dedicated sensors.
A very similar strategy is followed for the Mir space station, although in addition air samples are taken periodically in bottles for eventual analysis on the ground. This detailed offline analysis provides trend information only and cannot support a quick response to unexpected or sudden events.
For the International Space Station (ISS), a more sophisticated trace-gas monitoring system (TGM) is required. The long duration of the mission opens the door to excessive accumulation of off-gassed products, and the ever-changing payloads increase the risk of contamination through leaks. There is also the risk of unanticipated contaminants arising from, for example, uncontrolled microbial action. Early detection, identification and quantification of the release or build-up of harmful trace gases is therefore essential to safeguard the crew's health and safety.
The TGM strategy adhered to as a baseline during the early stages of the Columbus programme was a full on-board air analysis in real time covering all imaginable compounds, both qualitatively and quantitatively. It soon became clear that the equipment needed to achieve this was going to be too big, too heavy and too costly. It was therefore decided to step back and rethink the strategy. Was it really necessary, for example, to monitor all of the 300-plus trace gases on the SMAC list? A review of the results of atmosphere quality measurements from past Shuttle and Spacelab missions supported the view that, provided they were properly selected, the number of compounds needing to be monitored could be limited to about 20.
With this now much more manageable set of requirements, a search was made for a technique that had the potential to do the job whilst still being capable of being developed into a viable piece of space instrumentation. The Fourier Transform Infra-Red (FTIR) spectrometer was eventually chosen. The full details of the trade-off supporting this choice are beyond the scope of this article. Basically, however, most of the trace gases to be monitored are either inorganic or volatile organic compounds, the quantitative detection of which is the special province of FTIR spectrometry, a technique that also has high selectivity, adequate sensitivity and the potential to be engineered into a compact and robust instrument.
Figure 1 is a schematic of a typical FTIR spectrometer, which consists essentially of an infrared source, collimating and focusing optics, a Michelson interferometer for wave-length selection, a sample gas cell, and an infrared detector. During one measurement cycle, the 'moving mirror' is moved over a few millimetres (about 10), modulating the light from the infrared source. A plot of the intensity measured at the detector as a function of mirror position is the primary interferogram, which is the Fourier transform of the original spectrum of the light source. If a gas is introduced into the gas cell, the plot will contain the characteristic absorption features of the gas, enabling it to be identified.
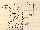
Figure 1. Layout of a typical FTIR system
Mixtures of gases can also be analysed in a similar way, but now the individual absorption features are all superimposed, resulting in very complex absorption spectra. Unravelling these spectra to identify the component gases and deduce their respective concentrations involves complicated data processing and is one of the greatest challenges of the technique.
Based on the results of analyses of the cabin air during several Shuttle and Spacelab missions, the decision was taken to concentrate on the 26 most frequently detected contaminants. These are listed, together with their corresponding 6-hour and 10-day SMAC values, in Table 1. Of these 26 compounds, just five proved particularly problematic for the FTIR and will need to be treated separately in most cases (e.g. for hydrogen and hydrazine) by using dedicated sensors. Detection of the remaining 21 compounds (items 1 21 in Table 1) therefore formed the driving requirement for the FTIR trace-gas monitoring system.
Table 1. Trace gases to be monitored
| No. | Gas | Formula |
10-day SMAC ppm |
mg/m³ |
6-hour SMAC ppm |
mg/m³ |
| 1 | Acetone | CH3-CO-CH3 | 200 | 475 | 750 | 1780 |
| 2 | Ammonia | NH3 | 25 | 18 | 25 | 18 |
| 3 | Benzene | C6H6 | 2 | 7 | 10 | 30 |
| 4 | Bromotrifluoromethane (Freon 1381) | CF3BR | 100 | 610 | 1000 | 6100 |
| 5 | 2-Butanone | CH3-CH2-CO-CH3 | 50 | 140 | 200 | 590 |
| 6 | Carbon monoxide | CO | 20 | 23 | 50 | 55 |
| - | Decamethyltetrasiloxane*¹ | SI4O3[CH3]10 | 15 | 115 | 70 | 480 |
| 7 | Dichloromethane | CH2Cl | 25 | 85 | 100 | 350 |
| 8 | Ethanol | CH3-CH2OH | 240 | 450 | 1000 | 1900 |
| 9 | Fluorotrichloromethane (Freon/Frigen 11) | CCI3F | 100 | 560 | 1000 | 5600 |
| 10 | n-Hexane | CH3[CH2]4-CH3 | 12 | 43 | 50 | 180 |
| - | Hydrogen*³ | H2 | 3000 | 245 | 12250 | 1000 |
| - | Hydrazine*² | H2N-NH2 | 0.004 | 0.005 | 0.015 | 0.02 |
| 11 | Methane | CH4 | 5000 | 3280 | 5000 | 3280 |
| 12 | Methanol | CH3OH | 50 | 60 | 200 | 260 |
| - | Monomethyl hydrazine*² | CH3-NH-NH2 | 0.01 | 0.02 | 0.03 | 0.06 |
| 13 | Nitrogen dioxide*4 | NO2 | 0.5 | 0.94 | - | - |
| - | Nitrogen tetroxide*5 | NO2O4 | 0.7 | 1.4 | 3 | 6 |
| 14 | 2-Propanol*6 | CH3-CH[OH]-CH3 | 50 | 120 | 500 | 590 |
| 15 | Toluene | C6H5-CH3 | 20 | 75 | 100 | 375 |
| 16 | 1,1,1 Trichloroethane | CCI3-CH3 | 80 | 450 | 350 | 1900 |
| 17 | Trichloroethylene | CHCI-CCI2 | 12 | 65 | 50 | 270 |
| 18 | 1,2-Trichlorotrifluoroethane (Freon 113) | CCI2F-CCIF2 | 100 | 760 | 1000 | 7600 |
| 19-21 | Xylenes*7 | C6H4-[CH3]2 | 20 | 87 | 100 | 435 |
1: not included due to too low a vapour pressure
2: not detectable within specified ranges
3: to be detected by separate fuel-cell sensor
4: only 10-day SMAC available
5: detected as N02/N2O4 equilibrium
6: SMAC values of 1-Propanol
7: three isomers: m-, o-, p-Xylene
A minimum detection threshold was defined for each compound, equal to 10% of the appropriate 10-day SMAC value. In addition, measurement accuracy had to be equal to or better than 10% throughout the concentration range defined by the 10-day and 6- hour SMAC values. All of these measurements must be made taking full account of the existence of carbon dioxide and water vapour as ever-present background gases. The time to be taken for one complete measurement was set at not more than 1 minute.
At this relatively early stage of development, the stringent requirements normally associated with the engineering of flight equipment were not made directly applicable. The ultimate purpose of the development was kept continuously in mind, however, resulting in the selection of a particularly compact and rugged FTIR interferometer and an emphasis on robust software able to cope not only with the analytical task, but also with inherent deficiencies in the FTIR technology, such as baseline drift.
In quantitative gas analysis, an important issue is the quality of the measured data, i.e. to what extent are the data reliable? High precision (i.e. good repeatability) does not necessarily imply good accuracy.
'Accuracy' is the ultimate quality criterion for any measurement because it determines how close an experimentally- determined value comes to the 'true' value. The difference between the two, i.e. the 'total analytical error', can be split into two contributions, the random error and the systematic error.
'Precision' is an estimate of the effect of random errors. If systematic errors are neglected, the requirement for 10% accuracy is a requirement for 10% precision, or repeatability, of measurement. This can be interpreted (ISO 6879 and ISO/DIS 9169.4) as the need for a measurement total standard deviation not exceeding 3.6% in the concentration range from 10-day to 6- hour SMAC values.
There are essentially three sources of random error: instrument defects ('hardware errors'), modelling inaccuracies ('software errors'), and calibration and test-gas errors ('calibration errors'). If the 3.6% standard deviation is, admittedly somewhat arbitrarily, split equally between these sources, each can contribute no more than 2.1% (in 'rms' sense). These individual contributions are discussed in a little more detail in the accompanying coloured panel (see next page).
The FTIR instrument consists of a 0.5 cm-¹ resolution Michelson interferometer, a multiple- reflection, variable-path-length 'White' gas cell in which the path length has been fixed at 8.94 m (equivalent to 16 passes) and an MCT (mercury-cadmium-telluride) semiconductor detector. The detector runs at a temperature of about 77 K and has its maximum sensitivity at a wavelength of 14 microns. The infrared source is a SiC glowbar working at a temperature of 910 K. The typically-achieved peak-to-peak signal-to-noise ratio (SNR) is about 500, and the stability of the instrument, measured in terms of the stability of the 100% line, is about 3%.
The instrument is shown schematically in Figure 2. Figure 3 is a photograph taken from above the instrument showing, from top to bottom, the interferometer, gas cell (left) and transfer optics (right), and liquid-nitrogen-cooled MCT detector (bottom right).

Figure 2. Schematic of the FTIR instrument: S = infrared source
D = detector BS = beam splitter
The flow-chart for a single measurement is illustrated in Figure 4. With the air sample in the gas cell, the primary interferogram data are recorded. After computation of the absorption spectrum via Fast Fourier Transform (FFT) analysis followed by reference-background division and logarithm computation, the concentrations of individual gases are evaluated by Partial Least Squares (PLS) multivariate analysis. The method employs a 'comb function' to systematically eliminate saturated or ambiguous regions of the spectrum from consideration, in order to cope with overlapping gas spectra and the spectral interference from the ever-present water vapour and carbon dioxide.
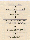
Figure 4. Measurement flow and data treatment
Baseline drift compensation
Instrument
instabilities are caused mainly by changes in source temperature
or by changes in the alignment of the interferometer. They are
manifested mainly via a drift in the 100% transmission line, the
so-called 'baseline'. In the prototype instrument, the drift was
a few percent over longish time periods and had to be compensated
for. Instead of making a baseline correction by pre-processing
and explicit fitting of baselines, an implicit baseline-drift
compensation was included in the model's construction and
calibration process.
The test results are summarised in Table 2 and Figures 5(a)-(d). Table 2 shows the accuracy achieved in the form of the standard deviation, and also lists the deduced detection limits calculated on the basis of the standard deviation for a 'zero-concentration' measurement (in accordance with ISO 6879 and ISO/DIS 9169.4).

Figures 5 a-d. Results of system performance tests (concentration
in ppm volume)
Tests with synthetic mixtures
Tests were
performed with 100 different synthetic gas mixtures. Table 2
(columns 3 & 4) lists the final results in terms of Standard
Error of Prediction (SEP) essentially, standard deviation
determined at the 10-day SMAC. The results have to be compared
with requirements (cf. 'S/W errors': SEP52.1% of 10-day SMAC
which is consistent with the required overall precision of 10%
of 10-day SMAC). It can be seen that the required standard error
criterion is met for all gases. The three most difficult gases,
toluene (2.07%), benzene (1.95%) and NO² (1.73%), are just
within the requirements. Benzene is interfering with carbon
dioxide, toluene is interfering with several other gases, and
N0² is interfering with water. This interference has two
effects: it lowers the effective signal-to-noise ratio due to
overlapping, leading to larger SEPs, and it generates non-
linearity effects due to insufficient resolution.
Table 2. Results of model testing with synthetic- and real-gas mixtures (1 min acquisition time)
No. Gas Standard Error of Standard Error of Detection
Prediction (Synt.) Prediction (Real) Limit
ppm %of 10-day ppm %of 10-day ppm Rel.to
SMAC SMAC Req.
1 Acetone* 1.38 0.69 0.99 0.50 2.3 0.12
2 Ammonia* 0.10 0.41 0.13 0.54 0.31 0.12
3 Benzene 0.04 1.95 0.066 3.3 0.15 0.77
4 Freon 1381* 0.17 0.17 0.15 0.15 0.34 0.034
5 2-Butanone 0.44 0.88 0.79 1.6 1.8 0.37
6 CO* 0.01 0.06 0.017 0.085 0.039 0.020
7 Dichloromethane* 0.073 0.29 0.15 0.62 0.36 0.14
8 Ethanol* 0.31 0.13 0.44 0.18 1.0 0.043
9 Freon 11* 0.34 0.34 0.36 0.36 0.83 0.083
10 n-Hexane* 0.06 0.50 0.31 2.6 0.71 0.61
11 Methane 1.50 0.03 8.2 0.16 19 0.038
12 Methanol** 0.06 0.12 8.4 17 - -
13 Nitrogen dioxide* 0.009 1.73 0.031 6.2 0.072 1.4
14 Isopropanol* 0.50 1.01 0.40 0.80 0.93 0.19
15 Toluene* 0.42 2.07 0.50 2.5 1.2 0.58
16 1,1,1-Trichloroethane 0.06 0.08 0.14 0.17 0.32 0.040
17 Trichloroethylene* 0.03 0.28 0.040 0.33 0.093 0.077
18 Freon 113* 0.15 0.15 0.26 0.26 0.61 0.061
19 m-Xylene* 0.11 0.53 0.079 0.40 0.18 0.092
20 o-Xylene* 0.09 0.47 0.086 0.43 0.20 0.10
21 p-Xylene* 0.05 0.27 0.10 0.51 0.24 0.12
22 Carbon dioxide 40.00 1.00 22 0.56 52 0.13
23 Water vapour** 34.80 0.13 6200 23 - -
Gases involved in the mixtures are in italics.
* Estimates for
standard error (real), and detection limit based on zero-
concentration measurements only.
** Estimates for detection limit
are not meaningful because of depletion due to gas-cell
adsorption.
Tests with real mixtures
Testing with
real gas mixtures was limited to the five compounds plus
background gases (water and carbon dioxide) listed in Table 3.
These gases are all among those for which real reference spectra
were measured, thus ensuring that the calibration was as accurate
as possible for these tests.
Table 3. Concentrations [ppm] of the constituents of the test mixtures according to the injected amounts
Mixture 2-Butanone Methanol 1,1,1- Benzene Methane Carbon Water
No. Trichloro- Dioxide Vapour
ethane
1 92.26 42.05 53.11 0.48 511 1014 4998
2 26.70 52.28 8.48 1.49 1217 2534 4998
3 65.42 66.78 13.70 4.21 1012 1517 7997
4 56.48 77.72 19.91 2.49 751 2017 7942
5 56.33 88.64 19.45 0.52 590 1012 5998
6 103.33 92.23 8.43 2.65 529 1512 5998
7 75.98 100.01 28.53 3.88 588 2009 6984
8 28.69 33.97 11.70 5.22 507 2517 6984
9 75.76 76.83 24.81 4.91 907 1007 4998
10 103.91 53.50 15.48 0.97 667 2007 4998
11 48.10 207 59.91 4.20 484 3992 4929
12 59.53 237 41.83 0.27 1001 3396 9996
13 12.47 193 57.33 8.80 643 2997 13419
14 24.18 226 72.02 2.56 821 1498 12050
15 5.96 225 49.95 0.23 1000 484 15062
Fifteen mixtures were made in order to test the system's overall analytical capability. The concentrations of the different gases in each mixture are shown in Table 3. The first four constituents and water were liquid when injected into the gas cell, whilst the methane and carbon dioxide were injected as gases. To minimise the uncertainty in the concentrations of the different gases, a liquid mixture of the above-mentioned (liquid) constituents was injected, instead of injecting small individual volumes of the pure liquids themselves. The amounts of liquid injected typically had relative standard deviations of less than 1%. The gases, methane and carbon dioxide, were injected with separate gas syringes.
The results of the tests with real gas mixtures are shown in graphical form in Figures 5(a) (d). The measured concentrations are compared with the reference (known) concentrations for four stable gases in the mixtures: benzene, 2-butanone, trichloroethane, and methane. Methane and water appeared not to be stable, due almost certainly to adsorption in the gas cell, and were disregarded. The solid line in each figure is the ideal line. In all cases, each of the fifteen gas-mixture measurements is actually represented by a cluster of four points, which are almost on top of each other and are the results of two 1-minute and two 5-minute measurements.
In general, there is good agreement between measured (predicted) and reference (known) concentrations. However, how reproducible the measurements are, in statistical terms, is better seen in Table 2. The SEP values in column four (measurements on real gas mixtures) for the compounds flagged with a single asterisk show only the results of instrument noise, baseline drift and some interference from nearby spectral lines, since their concentrations were zero in the gas mixtures concerned.
When the results are compared with the requirements the SEP for synthetic testing to 2.1%, the SEP for real gas testing to 3.6%, and the detection limit to 10%, all of the 10-day SMAC all are compliant except those for benzene and NO². From Figure 5b it can be seen that the smooth curve-fitting for benzene exhibits significantly larger deviations than appear among the groups of four measurements at each concentration. This can be explained by a nonlinear interference from carbon dioxide. In the case of NO², the high SEP is explained by interference from water. In both cases, the effect results from deviations from Beer's Law. A dedicated nonlinear-modelling exercise for these two compounds was successful in reducing the SEP for benzene from 3.3% to 2.2%, and that for NO² from 6.2 to 3.4%.
In an attempt to improve the performance still further, the effects of increased measurement time and refinements in baseline-drift compensation have been investigated. It was found that increasing the measurement time from 1 to 5 minutes resulted in an insignificant improvement. In the case of baseline-drift compensation, the spectra have been evaluated using background spectra obtained in two different ways. 'Measured background' means that each absorption spectrum has been calculated with a background measured a few minutes prior to measuring the spectrum. 'Common background' means that all the spectra have been calculated with one single background obtained earlier. This corresponds more closely to what will happen in practice in space, where the background will be measured only once per week or once per month. The common background in our case has been generated by taking an average of previously-measured backgrounds.
The test results showed that the baseline drift compensation was successful. In fact, the results showed that the use of 'common background plus baseline-drift compensation' typically produced better results than use of 'measured background'.
A prototype trace-gas monitor (TGM) based on FTIR technology has been developed to enable the quality of spacecraft cabin air to be monitored on board in near-real-time. The instrument makes extensive use of 'off-the-shelf' hardware and software and has demonstrated its ability to measure, reliably, at concentrations below 10% of 10-day SMAC values and in the presence of water vapour and carbon dioxide, the 21 trace contaminants most frequently found in the cabin air after Shuttle and Spacelab missions.
Further work will focus on refining the hardware and extending the library of detectable compounds.



 ESA Bulletin Nr. 89.
ESA Bulletin Nr. 89.