Solid and liquid fuel rockets
Which propellants are best? Remember that we want the exhaust jet to be moving as fast as possible. We can get a rough but informative answer from equations from kinetic theory and ideal gas theory:
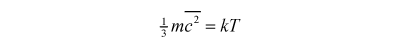
where m is the mass of the particles, 'c squared bar' the mean of the squared velocity of the particles, k the Boltzmann constant and T the temperature of the gas. So the 'root mean square velocity' of the gas particles is given by:
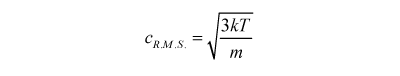
In other words the gas particles are moving fastest when they burn at a high temperature and are made of low mass molecules. A full analysis that will have to wait for a more advanced course gives us the velocity of the exhaust jet as:
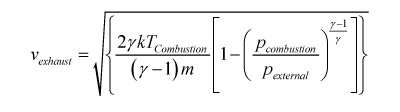
where γ is 'the ratio of the specific heats' (a constant between about 1.1 and 1.7 but dependant of the exhaust chemistry and temperature) and the 'p's are the pressure in the combustion chamber and outside the rocket. Clearly this is an unpleasant equation but it answers our simple question exactly the same way. We want a high combustion temperature and low mass exhaust particles.