Thrusters
Monopropellant systems
As the name implies monopropellant rockets do not carry separate fuel and oxidant supplies. Rather the propellant is a single liquid that decomposes to gases either on heating or when it is passed over a catalyst. There are two common monopropellants; hydrogen peroxide (H2O2) and hydrazine (N2H4). Hydrogen peroxide decomposes when it comes in to contact with a silver or platinum catalyst mesh (up to 98% concentration is used as a monopropellant):
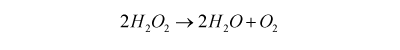
This produces steam and oxygen at over 600 °C and produces a specific impulse of up to 165 s. Hydrogen peroxide has also been used to power devices as diverse as drag racing cars and torpedoes.
Hydrazine is a more powerful but toxic monopropellant. It decomposes in contact with platinum/iridium metal into ammonia, nitrogen and hydrogen by a number of different possible paths:
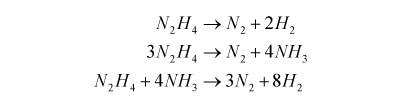
It does this at a temperature of around 800 °C and can produce a specific impulse of around 200 s.